
In the previous sections, nitrenium ion formation has been shown to be moderately influenced by electronic factors178,180,229 and steric factors have also been shown to play a small role.180 Electronic effects in butyloxy series 100 and benzyloxy series 151 are moderate, according to the results adumbrated thus far and they operate on the positive charge in the transition state for acid-catalysed solvolysis. A third series of compounds, benzyl N-benzoyloxybenzohydroxamates, was designed to investigate the electronic effects that substituents on the leaving group exert upon nitrenium ion formation.
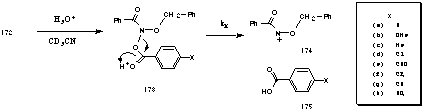
Benzyl N-benzoyloxybenzohydroxamates were prepared according to the general Method 2 described previously except that sodium benzoate salts were used instead of sodium acetate (Scheme 2-31).

The sodium benzoate salts 171a-h were generated from the appropriate benzoic acids with a 0.9 molar equivalent of sodium hydroxide solution, and the filtrate was collected from the suspension and thoroughly dried in an oven at 58oC before use. Pure benzyl N-benzoyloxybenzohydroxamates 172a-h were obtained by flash chromatography (hexane/ethyl acetate) in good to excellent yields. They were clear, viscous oils and could be stored under a blanket of nitrogen under refrigeration. Some crystallised over time. The compounds were however susceptible to hydrolysis and decomposed over several weeks. Thus they were used immediately after purification. Characterisation was through 1H and 13C NMR as well as IR spectroscopy.
Benzyl N-benzoyloxybenzohydroxamate 172a was solvolysed under acidic conditions in aqueous acetonitrile and in this series the progress of the reaction was monitored by integrating the disappearance of the benzyl methylene resonance over time according to the general 1H NMR method.

Benzyl N-benzoyloxybenzohydroxamate 172a decomposed by first-order kinetics as evidenced by the excellent relationship between ln[SM] and time (Figure 2-27). The acid-independent rate constants for the acid-catalysed solvolysis were determined from the kinetic runs over the temperature range 298-338K and an excellent Arrhenius plot was obtained (Figure 2-28).
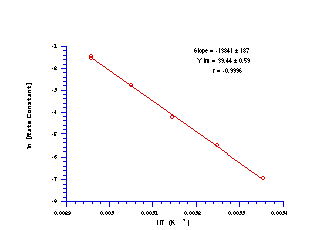
A least squares analysis of the solvolysis data gave the intercept as 39.44±0.59 which yielded a DS of +74.7±4.8 JK-1mol-1. The positive entropy change is consistent with release of benzoic acid concomitant with nitrenium ion formation (Scheme 2-32) but the change is lower than that found for the release of acetic acid from benzyl N-acetoxybenzohydroxamate (121±11 kJmol-1, see Section 2.3.4).
All members of the series of benzyl N-(para-substituted benzoyloxy) benzohydroxamates 172 were solvolysed under acidic conditions and the Arrhenius parameters were obtained from the acid-catalysed rate constants over the temperature range 298-338K. The results are reported in Table 2-13.
Table 2-13 Arrhenius parameters and rate constants for acid-catalysed solvolysis of benzyl N-(para-substituted benzoyloxy) benzohydroxamates 172a-h.
|
Subst |
|
|
|
|
|
|
|
|
172b MeO |
|
|
|
|
|
|
|
|
172c Me |
|
|
|
|
|
|
|
|
172a H |
|
|
|
|
|
|
|
|
172d Cl |
|
|
|
|
|
|
|
|
172e CHO |
|
|
|
|
|
|
|
|
172f CF3 |
|
|
|
|
|
|
|
|
172g CN |
|
|
|
|
|
|
|
|
172h NO2 |
|
|
|
|
|
|
|
Rate constants were obtained from single runs after adjusting for [H3O+]
Unlike the previous para-substituted benzyloxy series 151, DS and EA gave an excellent isokinetic relationship consistent with a single mechanism of solvolysis across the entire series.

The positive DS is in contrast to AAc2 hydrolysis of benzoate esters181 which show a strongly negative DS (acid-catalysed solvolysis of p-X-C6H4CO2Et in aqueous/organic medium are typically -100 to -120 JK-1mol-1). An increase in the electron-withdrawing ability of the para substituent led to an increase in the rate of solvolysis but the rate acceleration was not as great when compared to the effect of substituents on the solvolysis rates for the series of butyl N-acetoxybenzohydroxamates 100 (Table 2-2).
This is most probably a consequence of the hybrid nature of kH (kH = kK), the bimolecular rate constant. Substituent effects work in opposite senses for the protonation and heterolytic steps. While electron donor groups shift equilibrium protonation to the right, they would be expected to lower kH by destabilising a build-up of negative charge in the transition state. Conversely, electron-withdrawing substituents which would facilitate heterolysis would be expected to disfavour pre-equilibrium protonation. These counter electronic effects are reflected in the Hammett correlation.
The Arrhenius data was used to calculate the rate constants for solvolysis for each compound at 308K (Table 2-13). The data was found to give a poor correlation with s+ values (r= 0.862) but a reasonable relation with s was evident (Figure 2-30).

The positive slope is indicative of a transition state in which there is a developing negative charge on the leaving group. Heterolysis is promoted by electron-withdrawing groups.
Since the slope is positive, the influence of substituents upon the heterolytic steps is greater than upon the protonation step. Based upon the protonation at carbonyl of benzoic acids, r for this step should be in the region of -1 (although the solvent systems are different181). Hence a reaction constant for the heterolytic step would be in the region of +1.3 and entirely consistent with the build-up of negative charge at a centre indirectly conjugated to the substituent. The classical Hammett reaction constant for the benzoic acid-benzoate equilibrium is strongly solvent dependent, but in 50% aqueous ethanol is +1.52. Thus these results suggest a significant degree of benzoate character in the transition state.181
Finally, a positive reaction slope is further evidence against the normal AAc2 hydrolysis of the starting material involving attack at water at the protonated carbonyl moiety of the leaving group. Acid-catalysed hydrolysis of ethyl benzoates in aqueous ethanol proceeds with a similar insensitivity to substituents but r is a negative value.181 A bimolecular reaction involving solvent would be facilitated by electron-donating substituents which is clearly not the case.
It is clear that benzyl N-benzoyloxybenzohydroxamates undergo AAl1 solvolyses to give benzyloxynitrenium ions by analogy with N-acetoxy compounds. Arrhenius and Hammett data support this mechanism. Thus the AAl1 process appears to be general for alkyl N-acyloxybenzohydroxamates except where substituents on the alkyl group can strongly stabilise a benzyl carbenium ion (p-methoxy-, p-phenoxy-, p-phenybenzyl) in which case direct elimination of the nitrosocarbonylbenzene occurs preferentially.