
|
|
The Ames test is a salmonella-mammalian liver assay developed by Dr. Bruce Ames.245-248 The tester organism is the colon bacteria salmonella typhimurium, bearing a mutation that renders it unable to synthesise one of the enzymes responsible for the production of the amino acid histidine, which is a necessary component of proteins. As a result, the bacterium is unable to grow in a nutrient medium without an external supply of histidine. Spontaneous reversions occurring naturally restore the ability of the bacterium to produce the enzyme and allow the bacterium to form a colony. Chemicals that induce mutations increase the rate of change from the auxotrophic to the prototrophic form and this is reflected in an increase in the number of colonies that grow in the medium. Many chemicals are active mutagens only after metabolism by enzymatic processes in the body. To mimic the natural processes that occur in the liver, Ames added enzymes via an extract of rat-liver (S9) to the tester bacteria to convert the chemicals to their mutagenic metabolites. The enzymes are mainly cytochrome P-450 oxidases induced by deliberate administration of known carcinogens, such as Arochlor.
The rat liver extract, S9, is mixed with the tester bacteria and evenly spread on the agar medium. A DMSO solution of the suspected mutagen is added to the disk which is incubated for 2-3 days after which most of the histidine negative bacteria have died. Any DNA damage that results in histidine production caused by the chemical is detected as visible colonies. The mutagenic potential of the compound is proportional to the number of colonies produced. Direct acting mutagens yield positive test result without the addition of S9. Usually a linear dose-response is observed although at higher dose, toxicity may result in cell death.
The simplicity, sensitivity and accuracy249 of the Ames test for screening large numbers of potential environmental mutagens and carcinogens has resulted in its rapid acceptance as a standard procedure in many governmental, industrial and academic laboratories throughout the world.
Significant factors in the reactivity of alkylating agents is the accessibility to the nucleophilic sites on the DNA and the lypophilicity/hydrophobicity of the substrate.

The general cell structure for salmonella typhimurium is shown in Figure 4-1. As with all prokaryotic cells, the contents are protected by a relatively rigid cell wall. Within this wall is the cell membrane which is a very thin, highly flexible layer that is structurally very weak and it is through these layers that the food, waste and mutagenic compounds must pass. Most biological membranes contain phospholipids; compounds that contain highly hydrophobic and hydrophilic moieties which force the molecules to aggregate in a large micelle shell, called a phospholipid bi-layer. This thin, but highly specific bi-layer, is selectively permeable to small molecules such as alcohols, water, fatty acids and benzene which dissolve in the lipid phase of the matrix. Large polar molecules, such as sugars, amino acids and ions pass through the membrane through the action of membrane transport proteins. The phospholipid layer for the Gram-negative salmonella bacteria is relatively porous and consists of a complex polysaccharide called peptidoglycan.
Alkyl N-acyloxybenzohydroxamates 100, 151 and 172 are relatively small, non charged and non-hydroxylic molecules that should pass through the cell wall and membrane layers, however hydrolysis and/or phosphorylation by the phosphotransferase transport system could occur in the lipid matrix rendering the substrate inactive.
Within the cell and bounded by the cell membrane is the cytoplasm, a complicated mixture of substances bathed in water, in which the functions of the cell are carried out. The major components of the cytoplasm are macromolecules (proteins, nucleic acids, polysaccharides, lipids), ribosomes, small organic molecules (mainly precursors of macromolecules) and various inorganic ions. Prokaryotic DNA is not enclosed in a nuclear membrane but is found as a highly aggregated DNA molecule called the nucleoid which is extensively folded and twisted to fit into the bacterial cell.
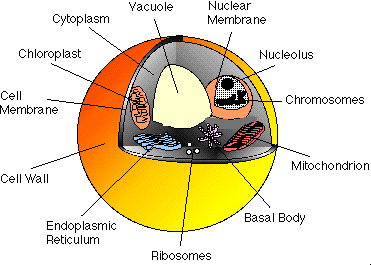
Eukaryotic cells are more complex inside the cell membrane, the genetic material being organised in chromosomes contained in the nucleus, a membrane-enclosed structure that functions as both a store house and factory for genetic information. The transport mechanisms across this lipid bi-layer are not as well understood, but are probably similar to the outer cell membrane.
Steric hindrance drastically limits the access of exogenous electrophiles to nucleophilic sites. While the phosphodiester groups and sugar hydroxyls are exposed to electrophilic agents, the double helix of DNA ensures that purine and pyrimidine bases are partially or totally hindered from attack. Generally, only nucleophilic centres situated on the major or minor grooves or in the walls of the double helix remain accessible to electrophilic attack. Recent advances in computational algorithms and processor power have allowed the reactivity of the DNA macromolecule to be examined by exploration of the electrostatic molecular potential in which high nucleophilicity is associated with the most prominent potential minima.250,251
The binding of chemical carcinogens to proteins has been the cause of much speculation and experimentation4 after Miller and Miller reported that carcinogenic compounds formed stable, covalently linked complexes with nucleic acids in vivo,65 however this binding alone is not sufficient to account for the chemical induction of cancer.
Intercalation of substrates with DNA is a fundamental process in which planar or aromatic functional groups of a substrate associate directly with DNA by fitting into the minor or major grooves of the helix. The driving force for the intercalation of aromatic substrates occurs through the lowering of the energy of the system with pi-pi stacking and is believed to be an important consideration in the process of mutagenesis for aromatic amines. The degree of intercalation is also intertwined with the lypophilicity of the substrate which determines the ease of transport through the cell to the DNA.
Alkyl N-acyloxybenzohydroxamates must pass through much cellular material to interact with DNA. Once through the cell membrane, the driving force for binding with hydrophobic DNA would probably be the hydrophobic nature of the mutagen and possibly pi driven intercalation. The later process is best if aromatics are electron deficient since this facilitates electrostatic attraction between electron rich (negatively charged) centres on the nucleotides and the electron deficient centres on the guest molecule. Thus acridines are outstanding intercalators although it is known that simple polycyclic aromatics may also intercalate effectively and the optimum size would appear to be three to four fused aromatics. Throughout the course of these studies, biological activity of substrates have been assayed by the Ames methodology. In this chapter the results of such assays are evaluated and point to some factors that may control the level of biological activity.
Mutagenicity testing was carried out at the Australian Commonwealth "Toxicology Unit of Worksafe Australia" in Sydney by Dr Antonio M. Bonin. Pure samples of the appropriate alkyl N-acyloxybenzohydroxamate were placed in sealed glass vials under an atmosphere of nitrogen gas. The samples were packed with dry ice and transported to the laboratories overnight and stored at -80 oC until the day of testing, when fresh DMSO solutions were prepared. The compounds were then subjected to the standard Ames test using TA100 salmonella typhimurium as the lawn culture. While TA98 and TA100 were used in preliminary tests, the latter was used as the reference strain to reduce costs, as both strains exhibited mutagenic responses when treated with DMSO solution of the mutagen. Metabolic activation was provided by a standard 10% S9 mix of Arochlor 1254-induced male (200-250 g) Sprague-Dawley rat livers.
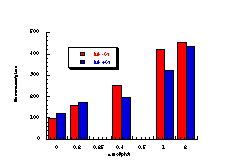
Butyl N-acetoxybenzohydroxamate 100a was one of the first alkyl N-acetoxybenzohydroxamates synthesised and subjected to the Ames test. Early "in house" mutagenicity testing with simple alkyl N-acetoxybenzohydroxamates (100a, 151a, 187 and 188) revealed that there was a positive induction of reversions and a linear dose-response relationship was evident in certain cases when moderate levels of mutagen (less than 200 µg/plate) were tested (Figure 4-4).172 The tests indicated that the mutagens were active in TA98 (susceptible to frame-shift mutations) and TA100 (susceptible to point mutations).252 In addition activity was evident with and without the administration of S9.172
|
|
|
Subsequently, data was obtained from the standardised tests performed by Dr Bonin who confirmed these trends in activity for butyl N-acetoxybenzohydroxamate 100a (Figure 4-5); at low doses a linear dose-response was evident which was similar in both the presence and absence of S9. The dose-response relationship became non-linear at higher dosages due to toxicity and resulted in a flattening of the response curve. As such, the dose-response relationship was calculated from the linear section of each curve. Since TA100 salmonella was found to give an acceptable dose-response curve and was more sensitive it was used for all further mutagenicity testing.
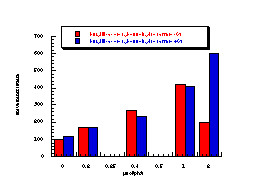
In dose-response plots such as these in Figure 4-5, the background reversion rate is available from the intercept and may be subtracted for the purposes of comparative studies. Interestingly, a linear dose-response relationship was observed when the concentration of applied mutagen without S9 activation, was less than 1.0 µmol per plate but above these doses the toxicity was evident with a diminution of revertants. The addition of the S9 agent however reduced the toxicity of the mutagen such that the linear dose-response was evident over the entire dosage tested. Neglecting the points after 1 µmol/plate for the mutagen without S9 activation allowed the dose-response at 1 µmol/plate to be calculated as the slope, which in this case was 319±30 (r = 0.9911).
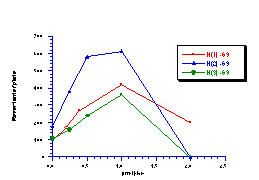
During the course of testing the butyl N-acetoxy (para-substituted)benzohydroxamate series, butyl N-acetoxybenzohydroxamate was tested independently three times with TA100 and the results are shown in Figure 4-6. The discrepancies are due to uncontrollable differences in the batches of TA100 bacteria which were cultured immediately prior to treatment with mutagen. Ideally a series of compounds should be tested from the same batch of salmonella for an accurate comparison but this was impractical in this study. Generally, samples from each series were synthesised, purified and dispatched in batches, several days or even several weeks apart. Therefore to offset the differences in responses between batches butyl N-acetoxybenzohydroxamate was used as the standard; responses for the standard enabled modification of data for new substrates by a factor based on the ratio of the activities of the standard at 1 µmol/plate in different batches.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|


(i)
(ii)
(iii)
(iv)
(v)
(vi)

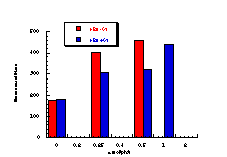
Figure 4-7(i-vii) show the dose-response graphs for the series of butyl N-acetoxybenzohydroxamates 100b-h over the range 0-2.0 µmol/plate with and without S9. All displayed a linear dose-response region when the concentration of applied mutagen was less than 1.0 µmol/plate but in some cases the number of revertants reduced above this dosage. For comparative purposes, S9 deficient test results were used. From the linear regions the slope for each compound was obtained and divided by the slope obtained for butyl N-acetoxybenzohydroxamate tested along with that particular set. The normalised slopes (with respect to butyl N-acetoxybenzohydroxamate 100a, from a linear dose region) are recorded in Table 4-4 and are presented in column graph format in Figure 4-8.
|
Subst. |
|
Corrected Slope¶ |
Normalised Slope |
|
|
100a H |
|
319(30) |
1 |
0.9981 |
|
100d Me |
|
323(22) |
1.0 (0.1) |
0.9953 |
|
100b MeO |
|
612(51) |
1.9 (0.2) |
0.9932 |
|
100a H |
|
812(7) |
1 |
0.9999 |
|
100c Ph |
|
2126(1) |
2.6(0.0) |
1.0000 |
|
100e But |
|
572(196) |
0.7(0.2) |
0.9459 |
|
100a H |
|
258(11) |
1 |
0.9927 |
|
100f Cl |
|
281(74) |
1.1(0.3) |
0.9372 |
|
100g Br |
|
397 (44) |
1.5(0.2) |
0.9940 |
|
100h NO2 |
|
122(9) |
0.5(0.1) |
0.9922 |

Mutagenicities across the series show no obvious trend. The ordering in Figure 4-8 corresponds to Hammett s substituent constants and reflects the electronic effects of para substituents. While the p-methoxylated mutagen 100b is more active and the p-nitro 100h is less active than parent 100a, the p-chloro 100f and p-bromo 100g compounds are clearly against the trend, whereas p-phenyl 100c is significantly more mutagenic than 100a. This cannot be due to electronic effects.
The ability of the substrate to mutate DNA is dependent upon several features; transport through the cell wall, electronic effects, hydrolysis, intercalation with DNA, specialised local pocket features, enzymatic destruction processes, etc. The biphenyl functional group could however increase activity through lipophilicity or hydrophobicity thus enabling more facile passage through the cell wall or increasing the binding with DNA (vide infra).
The mutagenicity levels for 151a-i were ascertained in TA100 salmonella typhimurium and are given in Table 4-5 to Table 4-7 and illustrated in Figure 4-9(i-ix).
|
µmol/plate |
PhO -S9 |
PhO +S9 |
Ph -S9 |
Ph +S9 |
NO2 -S9 |
NO2 +S9 |
|
0.000 |
175 |
182 |
175 |
182 |
175 |
182 |
|
0.250 |
805 |
474 |
1268 |
904 |
2090 |
444 |
|
0.500 |
0 |
1170 |
1751 |
1365 |
2153 |
788 |
|
1.000 |
0 |
676 |
2328 |
0 |
0 |
1105 |
|
2.000 |
0 |
0 |
2590 |
0 |
0 |
0 |
|
µmol/plate |
But -S9 |
But +S9 |
H -S9 |
H +S9 |
Br -S9 |
Br +S9 |
|
0.000 |
175 |
182 |
108 |
117 |
108 |
117 |
|
0.250 |
539 |
0 |
296 |
231 |
314 |
1419 |
|
0.500 |
626 |
0 |
427 |
557 |
0 |
2388 |
|
1.000 |
715 |
0 |
458 |
1187 |
0 |
2418 |
|
2.000 |
629 |
0 |
0 |
2015 |
0 |
0 |
|
µmol/plate |
Cl -S9 |
Cl +S9 |
MeO -S9 |
MeO +S9 |
Me -S9 |
Me -S9 |
|
0.000 |
175 |
182 |
175 |
182 |
175 |
182 |
|
0.250 |
543 |
1010 |
461 |
586 |
555 |
644 |
|
0.500 |
0 |
2383 |
821 |
923 |
682 |
1263 |
|
1.000 |
0 |
2626 |
0 |
1118 |
0 |
1230 |
|
2.000 |
0 |
0 |
0 |
1271 |
0 |
1334 |

(i)
(ii)

(iii)
(iv)
(v)
(vi)

(vii)
(viii)
(ix)
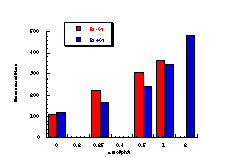
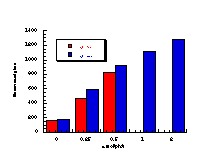
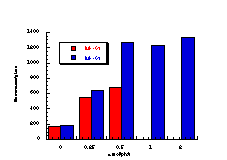
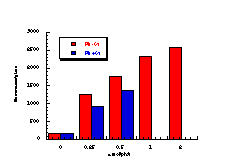
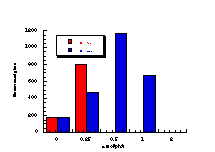
The raw mutagenicity levels revealed that apart from benzyl N-acetoxybenzohydroxamate, these compounds were significantly more mutagenic than the previous series but displayed higher toxicity towards the salmonella bacteria above 0.5 µmol/plate. Furthermore, while linearity could be detected at low dose, no clear trend in activity was evident. The dose mutagenicity data for benzyl N-acetoxybenzohydroxamate 151a displayed an excellent linear relationship when S9 enzymes were present (r2=0.995) over the range 0-2 µmol/plate whereas in the absence of applied enzymes the response at higher doses was modified by toxicity (Figure 4-9i). At 2.0 µmol/plate the compound displayed no net mutagenicity (Figure 4-10).
Similar results were found for other compounds (Figure 4-9(ii-ix)).

To enable some comparison to be made, the data for all compounds was extrapolated back to the 0.25 µmol/plate level where reasonable linear-dose relationships held. Furthermore to allow comparison with the results obtained for the previous alkyl N-acetoxybenzohydroxamate series 100 the measured mutagenicity levels are normalised with respect to the standard compound, butyl N-acetoxybenzohydroxamate 100a, which was tested in parallel with these batches. A comparison of the normalised mutagenicity levels for this series without S9, is shown below (Figure 4-11).
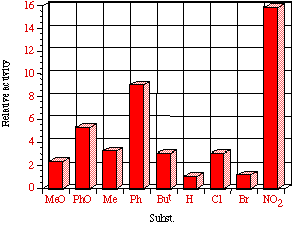
Across the electronic series it is evident that no clear trend is apparent. The data for para-phenoxy 151b and para-phenyl 151d substrates indicate greater activity relative to the unsubstituted compound. Once again, aromatic character would appear to enhance mutagenicity. It is significant that, while studies outlined elsewhere in this thesis indicate that these two substrates 151b and 151d together with p-methoxy mutagen 151c do not form nitrenium ions even under acid-catalysis, but rather generate pare-substituted benzyl cations, they are nonetheless significantly mutagenic. Either the actual chemical interaction with DNA leading to the mutagenic activity occurs by an alternative process or the substrates behave as alkylating agents through benzyl cation formation. The extreme mutagenicity of the p-nitro compound 151i is interesting and will be addressed in Section 4.5.2. In this series, S9 appeared to enhance activity and particularly so in the case of the p-chloro 151h and the p-bromo 151g substrates. The role of S9 in this enhancement as well as in the general detoxification of substrates is beyond the scope of this study.
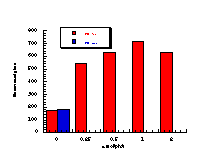
The mutagenicity of a series of benzyl N-benzoyloxybenzohydroxamates 172a-e, 172g and 172h, was measured for dose levels of 0 - 0.25 mmol/plate in TA100, with and without enzymatic activation. The low levels of applied mutagen were employed to ensure adequate mutagenicity levels without toxicity to the bacterial strain.
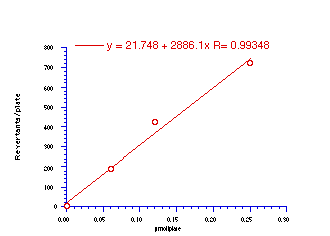
Figure 4-12 displays the dose-response relationship for 172a and reflects the characteristically excellent results obtained for this series of compounds. Table 4-8 and Table 4-9 record the results for 172b-e, 172g and 172h.
|
µmol/plate |
Stand -S9 |
H -S9 |
MeO -S9 |
MeO +S9 |
Me -S9 |
Me +S9 |
|
0.00 |
0(8) |
0(8) |
0(10) |
0(7) |
0(10) |
0(8) |
|
0.03 |
|
|
27(9) |
100(12) |
40(10) |
86(5) |
|
0.06 |
|
188(12) |
51(11) |
154(14) |
127(6) |
158(17) |
|
0.12 |
|
420(24) |
156(37) |
296(7) |
328(150) |
371(10) |
|
0.25 |
78(4) |
720(78) |
324(77) |
696(59) |
1286(38) |
1008(67) |
|
0.50 |
355(59) |
|
|
|
|
|
|
1.00 |
448(54) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(i)
(ii)

(iii)
(iv)

(v)
(vi)
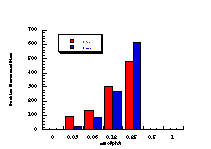
The data in Table 4-8 and Table 4-9 are the positive dose-responses for benzyl N-benzoyloxybenzohydroxamates 172a-e, 172g and 172h in TA100 salmonella typhimurium after the background reversion rate has been subtracted. Each dose level was tested with three plates of bacteria and the average level and population standard deviation errors (sn) were calculated. Butyl N-acetoxybenzohydroxamate 100a was tested together with the series and the linear dose-response region is described by: Slope = 474±119; Y Intercept = 13±68.412; and r = 0.9421. Dose-response plots for each substrate are given in Figure 4-13(i-vi).
The linear dose-response region for each compound (-S9) was calculated and normalised, with appropriate analysis of errors to give the normalised response relative to butyl N-acetoxybenzohydroxamate 100a (Table 4-10). The data is presented in column graph format in Figure 4-14.
|
Substrate |
Normalised Mutagenicity |
Error |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
†based on 100a displaying 474 positive reversions/plate.

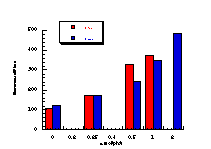
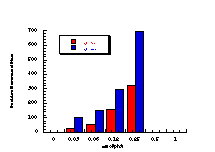
Figure 4-14 illustrates that the most mutagenic compounds were the para-methyl 172c and parent benzyl N-benzoyloxybenzohydroxamate 172a, while the least mutagenic were the p-nitro 172h and p-methoxy 172b compounds respectively. Excluding the p-methoxy results, mutagenicity would appear to increase with electron-donor capacity of the para substituent (Figure 4-15).
The quality of the data obtained for this series and the apparent trends in Figure 4-14 was encouraging and justified further qualitative analysis.
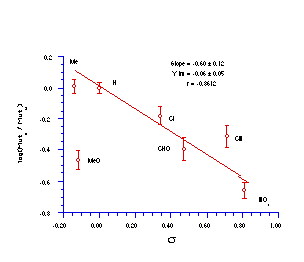
The relative mutagenicities appear to correlate with Hammett s substituent constants (Figure 4-15) with a low but significant r value of -0.60±0.12 (r=0.86). It is noteworthy that both nitrenium ion formation in acid-catalysed solvolysis (r=+0.32), as well as SN2 reactivity with base (r= +0.55) and on aromatic amines244 (r=+1.3) yield positive correlations in the same series of compounds. The activity therefore appears to be correlating with stability. An interpretation of the above might be that the lower the reactivity, the higher the probability that the mutagens survive to reach the target sites of DNA or other receptors.
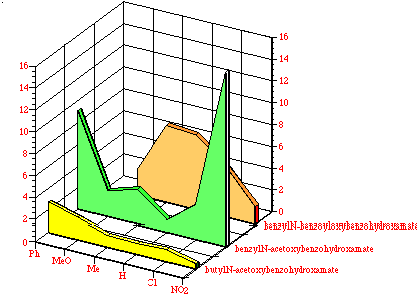
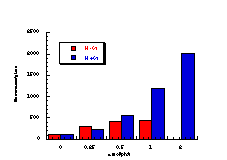
While comparisons, when common para substituents are present, are difficult across the three benzohydroxamate series the average mutagenicity tended to increase from butyl N-acetoxybenzohydroxamates 100 through benzyl N-acetoxybenzohydroxamates 152 to benzyl N-benzoyloxybenzohydroxamates 172. This can be seen in Figure 4-16 which compares the normalised mutagenicity (as area) with structure. Qualitatively, as the hydrophobicity increases with the number of phenyl rings, the area under the curve similarly increase.
In addition, in the two cases where p-phenyl substituents were tested, 100c and 151d, there is a significant increase in the mutagenicity when compared to the corresponding unsubstituted substrate, 100a and 151a, respectively. The biphenyl group would be expected to increase the hydrophobicity of the mutagen relative to phenyl.
The moderate mutagenicity of butyl N-acetoxy-p-nitrobenzohydroxamate 100h is interesting in that there is a known alternative mechanism by which arylnitro groups can cause mutations.
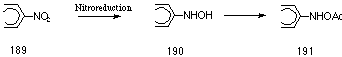
Nitro-polycyclic aromatic hydrocarbons (nitro-PAHs) are among the most potent mutagens as determined by the Ames tests with salmonella typhimurium. The genotoxicity of nitro-PAHs has been extensively reported253 and the accepted mode of metabolism involves reduction of the nitro group 189 to nitroso-, N-hydroxylamino-, and amino derivatives. Of these metabolites, the N-hydroxylamino intermediate 190 is believed to be responsible for the carcinogenicity and mutagenicity and can be further activated via O- 191 and N-acetylation (Figure 4-17). The active electrophilic metabolites from the reduction of nitro-PAHs are thus similar to those formed from the oxidation of aromatic amines.
The mutagenic activity of the nitro-PAH compounds is strongly influenced by several structural factors such as isomeric position of the nitro group, conformation of the nitro group with respect to the plane of the aromatic ring, physical dimension of the aromatic rings and the ability to resonance stabilise the electrophilic metabolites.
The mutagenic activity of butyl N-acetoxy-p-nitrobenzohydroxamate 100h was tested in TA100 Salmonella typhimurium which is a strain of bacteria that contains nitroreductase components that efficiently reduce nitro-PAHs.254 Our results indicate that the nitro group is not reduced by the reductase in this strain as this compound displayed the least activity of the series. Nitroreductase activation would have greatly increased the mutagenic potency of this compound but this was not evident. The electron-withdrawing carbonyl moiety para to the nitro group may impede nitroreductase activity or, alternatively, reduce the electrophilicity of intermediates from metabolic modification.
It is perhaps significant that the p-nitrobenzoyloxy substrate 172h also exhibited modest mutagenicity relative to the rest of the series. Here too the nitro group is para to an electron deficient carbonyl. In the case of the benzyloxy series however, the p-nitrobenzyl N-acetoxybenzohydroxamate 151i is extremely mutagenic when compared to the unsubstituted member of that series. Here, the para-nitro aromatic moiety is most probably far less deactivated by the oxymethylene para substituent. Further experiments with substrates bearing meta-nitro groups or nitro-reductase-free TA100 would assist in explaining these anomalous results.
Every member of this new class of alkyl N-acyloxybenzohydroxamates tested to date is mutagenic. There is no clear cut correlation with electronic effects of substituents when the benzoyl 100 and benzyloxy 151 series are considered.
In the case of the benzoyloxy series 172, the mutagenicity can be regarded as correlating with the inverse of reactivity and hence correlating with stability. Recent results from these laboratories have consolidated this correlation.255
Several factors point to hydrophobicity as an activating factor. Mutagenicities generally increase across the series 100 through 151 to 172 (that is, with increasing aromatic content). Biphenyl substitution also markedly enhances activity relative to the corresponding phenylated substrate. Recent results also show that a butyl N-acetoxy-2-naphthohydroxamate 192 (5500 revertants at 1 µmol/plate), is more than ten times as active as 100a (477 revertants at 1 µmol/plate).209

DNA damage studies230 have recently indicated that
alkyl N-acyloxybenzamides react at N-7 of guanine and, as
such, must react in the major groove of DNA in which this, the most
nucleophilic site, is exposed to electrophiles. Thus the observed
increase in activity with hydrophobicity may be related to the
increased capacity of these substrates to bind hydrophobically in the
major groove of DNA.